Abstract
[Backgrounds] Intravascular large B cell lymphoma (IVLBCL) is a rare subtype of extranodal diffuse large B-cell lymphoma (DLBCL) that features lymphoma cells lodged in lumina of small vessels. Because of the lack of mass formation, diagnosis by tumor biopsy is often difficult, and thus, cell-free DNA (cfDNA) analysis might be useful for assisting diagnosis of IVLBCL. In this study, we first aimed at investigating the prevalence of mutations in genes involved in the B-cell receptor, Toll-like receptor/interleukin-1 receptor, and nuclear factor kappa B pathways. We then evaluated the liquid biopsies for detection of hot-spot mutations by droplet digital polymerase chain reaction (ddPCR).
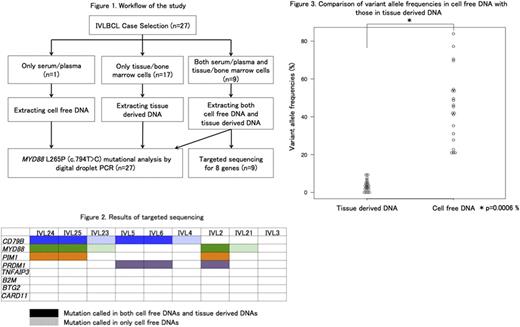
[Patients and Methods] Twenty-seven IVLBCL patients whose archived samples were available were included in this study. The patients were diagnosed as IVLBCL from January 2005 to May 2017 in University of Tsukuba Hospital, JA Toride Medical Center, and Kameda Medical Center. The study was approved by the review board in each institution, and conducted according to the Declaration of Helsinki. Tissue-derived DNA (tdDNA) was extracted from formalin-fixed paraffin-embedded specimens and/or cryo-preserved bone marrow samples, in which the lymphoma cell infiltration had been pathologically confirmed. cfDNA was extracted from 1 ml of serum or plasma obtained prior to chemotherapy. We performed targeted sequence for 8 genes (B2M, BTG2, CARD11, CD79B, MYD88, PIM1, PRDM1, and TNFAIP3) in paired tdDNA and cfDNA from 9 IVLBCL subjects by Ion Torrent Personal Genome Machine (Thermo Fisher Scientific). MYD88 L265P (c.794T>C) mutational analysis was performed by ddPCR assays (BioRad) for 46 samples in total collected from 27 subjects. Statistical analysis was performed with EZR version 1.3.2. Paired t-test was used to compare variant allele frequencies (VAFs) in paired samples.
[Results] All cases were categorized as the non-GCB by Hans classification. Random skin biopsies (RSB) and bone marrow biopsies (BMB) were performed in 21 and 27 patients. The diagnostic yield of RSB was 81.0% (17 of 21). Initial BMB detected lymphoma cells in 48.1% (13/27) patients, but repeated BMB increased successful detection up to 66.7% (18/27). tdDNA was available in 26 out of 27 subjects, and in 9 of which paired cfDNA was also available (Figure 1). In one subject, only cfDNA but not tdDNA was available. Targeted sequencing for 8 genes demonstrated at least one mutation in CD79B, MYD88, PIM1, or PRDM1 in both tdDNA and cfDNA, or only in cfDNA, in 8 of 9 subjects (Figure 2). We identified 3 missense CD79B mutations in 6 (66.7%; Y196C in 2, Y196H in 3, and L199P in 1). All MYD88 mutations were L265P, which were found in 5 of 9 (55.6%). Together, either Y196 CD79B or L265P MYD88 mutation was found in 7 of 9 (77.8%). PIM1 mutations and PRDM1 mutations were seen each in 3 (33.3%). VAFs were higher in cfDNA than in tdDNA (mean, 45.6% vs. 3.7%; P=0.0006, Figure 3). Four mutations in 3 patients were detected only in cfDNA. On the other hand, all variants detected in tdDNA were confirmed in cfDNA. ddPCR assay for the L265P MYD88 mutation with all 46 samples including tdDNA and cfDNA revealed the L265P MYD88 mutation in 24/46 (52.2%) samples from 16/27 (59.3%) IVLBCL patients. Furthermore, sequential analysis of cfDNA was performed in one of the MYD88 -mutated patients. The L265P MYD88 mutation was detected (VAF=30%) in cfDNA collected 17 weeks before the diagnosis of IVLBCL, whereas the mutation was not detected in DNA derived from the bone marrow sample that was collected at the same timing and resulted in the negative pathological finding.
[Conclusion] Mutations in MYD88 and CD79B are frequent in IVLBCL . Targeted sequencing suggested that tumor cell-derived DNA is abundant in serum/plasma. Liquid biopsy to detect L265P MYD88 and Y196 CD79B may provide a powerful tool for approaching diagnosis of IVLBCL.
Chiba:Nippon Shinyaku: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal